Farm animals are often exposed to mycotoxins, which have many adverse health effects and are becoming a major food safety challenge. Understanding how the mycotoxin interact with the organism can provide useful information into their mechanism of action, thereby facilitating the development of strategies to reduce the harmful effects of these compounds in animal nutrition, such as the inclusion of detoxifying agents into the diet to mitigate the mycotoxins exposure. However, following the European Food Safety Authority recommendations, the efficacy of detoxifiers should be assessed through toxicokinetic studies. Toxicokinetic studies describe the fate of a toxin in the body, depending on absorption, distribution, metabolism and excretion (ADME) processes.
Gruberg- Dorninger et al. (2019), showed that in poultry species, the main mycotoxins found in contaminated feeds are fumonisins (FBs), which account for 60% of the contamination in finished feeds. It has been reported that in broiler chickens, FBs are absorbed in approximately 2-3% in the gastrointestinal tract, exhibiting poor bioavailability and rapid distribution in tissues, such as the liver, kidney, and muscle (Knutsen et al., 2018). Regarding their metabolism, literature describes hydrolysis reactions that lead to the production of the two main metabolites, PHFB1 (partially hydrolyzed FB1) and HFB1 (hydrolyzed FB1) (Knutsen et al., 2018).
Also, it has been shown that FBs are structural analogues of sphingoid bases that inhibit ceramide synthase. This inhibition lead to disruption of sphingolipid metabolism and pathological changes. In fact, the alteration of the sphinganine/sphingosine ratio is considered a biomarker of great interest to determine the toxicity of fumonisins (Guerre et al., 2022).
Concerning the excretion of FBs, the primary pathways involve the biliary duct, with a higher elimination observed in feces, typically accounting for 90% of the administered dose. Both FBs and their metabolites have been identified in fecal samples, exhibiting a half-life of less than 4 hours (Knutsen et al., 2018). Consequently, toxicokinetic studies, based on ADME processes, are useful tools to evaluate the susceptibility of animal species to mycotoxins and to evaluate the efficacy of anti-mycotoxins solutions.
BIŌNTE® QUIMITŌX® PLUS®: FB1 Toxicokinetic trial in broiler chickens
BIŌNTE® QUIMITŌX® PLUS® combines adsorbent materials to effectively bind mycotoxins in animal nutrition. Additionally, it contains a blend of natural extracts and selected yeasts, offering bioprotective and post-biotic effects. This unique combination of minerals, organic materials and phytogenic components, has been developed by a multidisciplinary scientific team in strict compliance with EFSA requirements.
To address this issue, and provide useful guidelines to the sector, an in vivo trial was carried out in collaboration with Ghent University to evaluate the effects of BIŌNTE® QUIMITŌX® PLUS on the oral bioavailability of FB1 in broiler chickens evaluating the plasma concentration-time profile.
As shown in figure 1, the trial involved six 21 day-old-broiler chickens Ross 308 which were fed with a commercial diet from day 1-6 (acclimatization period). This feed was naturally contaminated with low levels of deoxynivalenol (152 ppb), zearalenone (19.9 ppb) and FB1+FB2 (51.2 ppb).
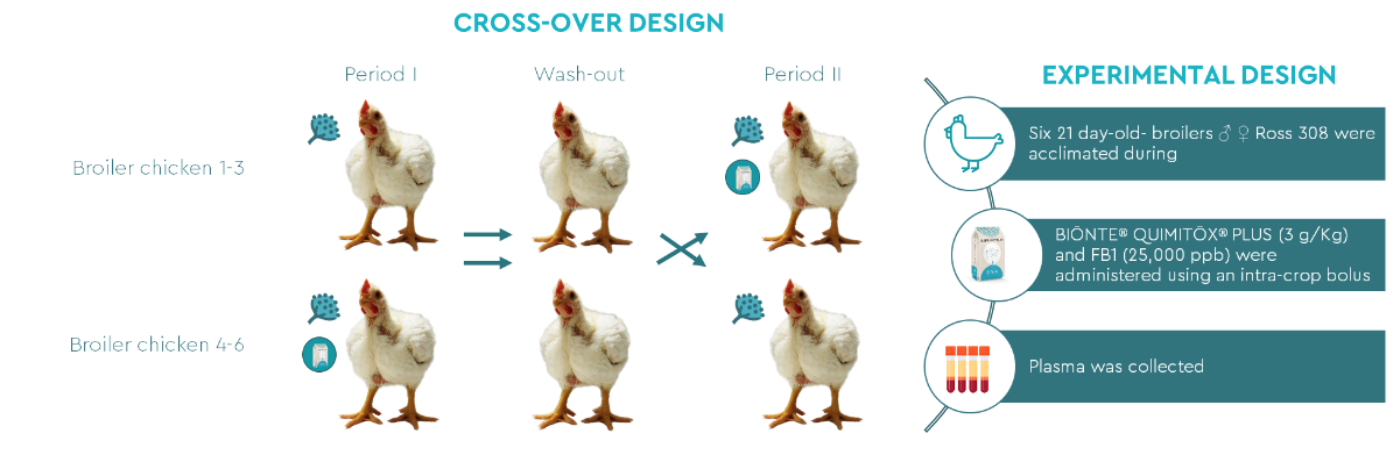
Figure 1. Experimental design.
Following the experimental design, at period I, 3 broilers chickens were administered a single oral bolus with 25000 ppb of FB1, while the others 3 animals received the same single oral bolus of FB1 (25000 ppb) in combination with 3 g/kg of BIŌNTE® QUIMITŌX® PLUS. At period II, a cross-over design was applied for the administration of the mycotoxin and the mycotoxin combined with anti-mycotoxins solutions for the different broiler chickens. Blood samples were collected before the administration of treatments and at 0.08, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 8 and 12h after the administration.
Toxicokinetic analysis included the examination area under the plasma concentration- time curve from time 0 to 12h (AUC 0→12 ) and maximum plasma concentration (C max ) among other toxicokinetic parameters. The effect of BIŌNTE® QUIMITŌX® PLUS on the oral absorption of FB1 was assessed by comparing toxicokinetic parameters based on FB1 plasma concentrations in samples treated with FB1 alone versus those treated with FB1 combined with the tested anti-mycotoxins product, with a focus on AUC 0→12 and C max .
Trial results: Efficacy of BIŌNTE® QUIMITŌX® PLUS
The systemic exposure (reflected by the AUC) of FB1 was statistically significantly lower in the birds receiving BIŌNTE® QUIMITŌX® PLUS, compared to the birds that only received the mycotoxin (Figure 2). More specifically, the AUC 0→12 and C max showed a reduction of 82% and 74%, respectively (Table 1).
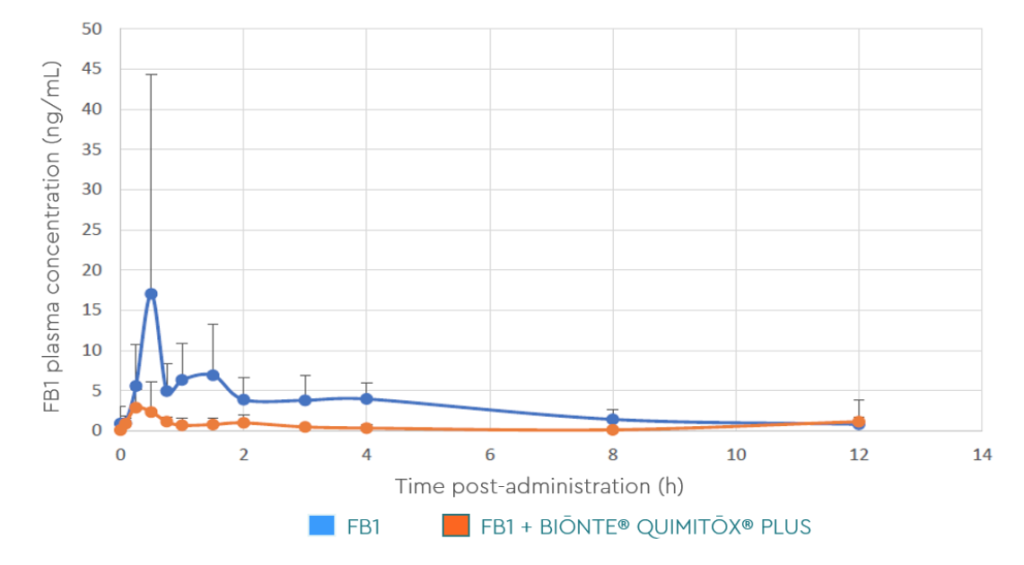
Figure 2. Plasma concentration-time profile of FB1 after a single oral bolus administration of 2.5 mg
FB1/kg BW in combination with or without 3.0 g BIŌNTE® QUIMITOX® PLUS®/kg BW.
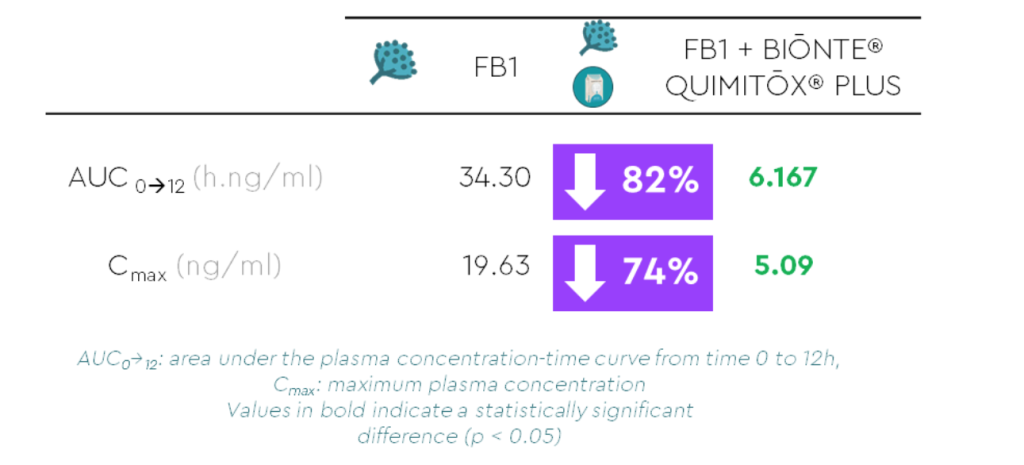
Table 1. Main toxicokinetic parameters of FB1 after an oral bolus administration, whether or not combined with BIŌNTE® QUIMITŌX® PLUS.
These results, published in the World Mycotoxin Forum (2023), showed the efficacy of BIŌNTE® QUIMITŌX® PLUS in reducing exposure to FB1 in broiler chickens.
This trial provide insight into plasma concentration-time profile of FB1 in broiler chickens, demonstrating the efficacy of BIŌNTE® QUIMITŌX® PLUS in reducing the total systemic exposure to FB1 in this specie.



