Biomarkers in the food safety field are defined as a measurable and evaluable characteristic that is indicative of a normal metabolic process, a pathogenic process or a biological response to a therapeutic treatment (ACSA, 2023). Among the different types of biomarkers, biomarkers of exposure stand out for allowing the assessment of an individual’s exposure to toxins or other agents by determining their metabolites in biological matrices such as blood plasma, liver tissue and excreta/feces (Vidal et al., 2018; Lowry, 1995). Characterized biomarkers of exposure have been shown to predict relevant clinical outcomes in a variety of treatments and populations (Atkinson et al., 2001). In fact, nowadays, the use of biomarkers has become common, and scientific research based on biomarkers has gained recognition (Vidal et al., 2018).
Biomarkers of exposure: Mycotoxins and their metabolites
The negative effects of mycotoxins differ greatly within animal species; therefore, the study of conclusive biomarkers is a direct diagnosis tool in the field. In 2018, the European Food Safety Authority (EFSA) established recommendations for the assessment of exposure to mycotoxin contamination by determining the most relevant metabolites for the main mycotoxins and biological matrices. Therefore, mycotoxin exposure in animal nutrition should be monitored by measuring the appropriate biomarkers relative to each mycotoxin and target species (Lauwers et al., 2019). The table below describes the main biomarkers for the evaluation of exposure to mycotoxin contamination, according to EFSA (2018).
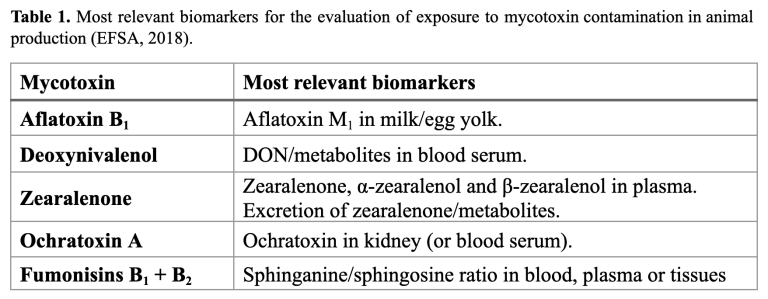
Efficacy demonstration
Anti-mycotoxin agents are products designed to mitigate the mycotoxins present in the diet in order to protect the animal’s health. However, they do not develop their action until the feed is ingested. Despite carrying out in vitro efficacy studies against different mycotoxins simulating the conditions of the gastrointestinal tract, which are very useful for the research of new materials and products, the final demonstration of efficacy must be based on in vivo studies (EFSA, 2018). In this context, the European food safety administrations established that the efficacy of anti-mycotoxin additives should be demonstrated by the reduction of mycotoxin adsorption in fluids and tissues (plasma, liver, etc.), the increase in excretion of mycotoxins (feces and excreta) and the reduction of contamination in food of animal origin (milk and eggs) (EFSA, 2010; EFSA, 2018). Consequently, biomarkers of exposure play a key role in evaluating the efficacy of anti-mycotoxin agents by monitoring mycotoxins and their metabolites in different biological matrices (Lauwers et al., 2019).
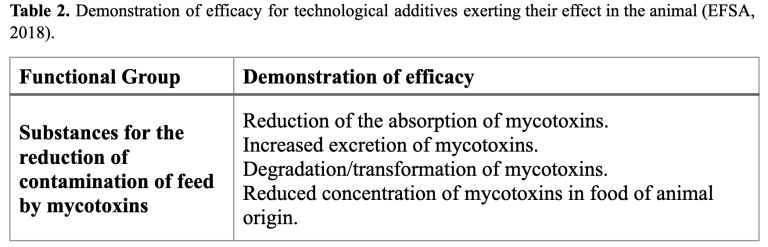
Advantages and disadvantages of the analysis of mycotoxins and their biomarkers in biological matrices
The analysis of mycotoxin biomarkers in biological matrices is a direct tool for the diagnosis of exposure to mycotoxins in the field. However, the biomarkers must be specific for each mycotoxin and target species. In addition, the analysis method for their detection must be validated for each biological matrix of study (EFSA, 2010).
Mycotoxins are a wide group of chemical compounds with very different properties. Therefore, they present different kinetic profiles of adsorption and metabolization in the body of each animal species of interest (EFSA, 2010). These distinctions must be considered when choosing the biological matrix to study and, consequently, when establishing sampling guidelines (Vidal et al., 2018; Lauwers et al., 2019).
In blood plasma, sampling time is critical and must be strictly planned according to the last feed intake. The maximum absorption point of the mycotoxin of interest in the blood should be considered. This sampling plan is not viable for most farms, and procedures that are not sufficiently controlled could lead to the misinterpretation of the results and the underestimation of mycotoxin contamination risk. For this reason, it is important to establish a very controlled feeding and blood collection protocol (Lauwers et al., 2019).
On the other hand, in the liver or kidneys, mycotoxins bioaccumulate before being eliminated from the body (Escrivà et al., 2017). Consequently, they are biological matrices less subject to the variability induced by feed intake and sampling practices. However, the sampling is an invasive procedure that may not be feasible without the animal sacrifice.
Finally, the analysis of mycotoxins and their metabolites in excreta/feces is critical to demonstrating the efficacy of anti-mycotoxin products. The mycotoxins captured by the anti-mycotoxin agents in the gastrointestinal tract are eliminated from the body through excreta/feces, showing an increase in the concentration of toxins in this matrix compared to animals without treatment (EFSA, 2010; EFSA 2018). Furthermore, taking excreta/feces samples does not require animal handling, so it facilitates the implementation and management of the program on the farm. For these reasons, excreta/feces analysis is a direct and specific tool to evaluate the efficacy of the strategies to mitigate the impact of mycotoxin contamination in animal production.
The challenge of correlating feed and biological matrices analysis
Given the heterogeneity of the occurrence of mycotoxins in feed and the differences in the toxicokinetics of each target species, it is extremely difficult to predict how the feed contamination will affect the animal and vice versa. The assays require expert laboratories equipped with liquid chromatography with mass spectrometry (HPLC-MS/MS) for the determination of mycotoxins and their biomarkers at a trace level. Furthermore, due to the high complexity of the samples, new extraction methodologies must be developed and validated for each mycotoxin, metabolite and matrix (EFSA, 2010). Currently, biomarkers of exposure to mycotoxins are a valuable diagnostic tool; however, more knowledge is required about the bioavailability and toxicokinetics of mycotoxins to establish a direct correlation with contamination in feed (Vidal et al., 2018; Lauwers et al., 2019).
Mycotoxins and their biomarkers analysis service in excreta/feces
BIŌNTE offers the service of analysis of mycotoxins and their metabolites in excreta/feces to detect and quantify the exposure to mycotoxins in the field using the HPLC-MS/MS methodology. The analysis of the biomarkers of the main mycotoxins, AFB1, DON, ZEN, OTA, T-2 and FB1+FB2, is performed by an accredited laboratory. The excreta/feces analysis service is part of BIŌNTE’s technical service portfolio (BIŌNTEX) and includes expert advice on the diagnosis of mycotoxin risk and the implementation of corrective actions to mitigate the negative effects of mycotoxins on animal production.
Conclusion
The analysis of biomarkers of exposure to mycotoxins in excreta/feces is a direct and specific tool to diagnose and monitor the real impact of mycotoxins in the field and demonstrate the conclusive efficacy of different mycotoxin mitigation strategies.



