Introduction
The genus Penicillium, widely distributed in the environment, comprises about 350 species, known for both their industrial applications and their ability to produce mycotoxins, secondary metabolites toxic to mammals and other animal species.
The toxins made by Penicillium are produced in both field and storage conditions. Among these, ochratoxin A (OTA) is one of the most relevant due to its renal toxicity, carcinogenic, teratogenic and immunosuppressive properties.
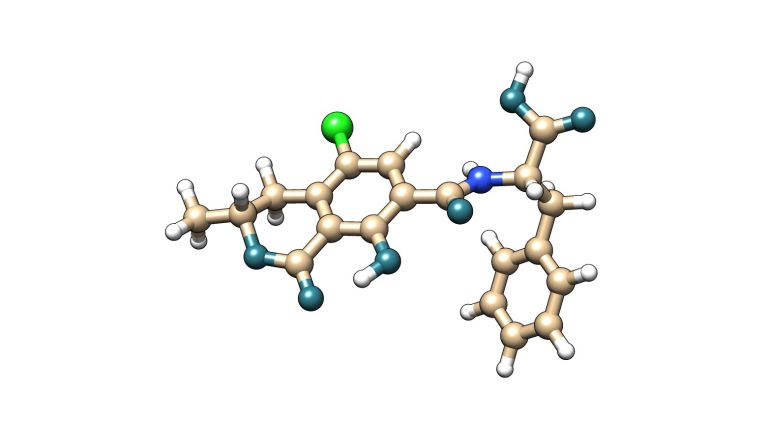
Image 1. Ochratoxin A (OTA) molecule
Morphological characteristics
The characteristic structure of Penicillium spp. has a brush-like shape which gives the genus its name (from the Latin Penicillus, ‘small brush’)). In Penicillium verrucosum, the following parts are distinguished:
- Conidia: Asexual spores produced in the terminal part of the philalides. The conidia are small, spherical or elliptical.
- Phialide: Specialized cell in the production of conidia, in the form of elongated bottles and are directly connected to the metulae.
- Metulae: Branched structures that support the phialides.
- Stalk: Extensions of the mycelium that carry the reproductive structures, that can be divided into primary and secondary branches.
- Stipe: Long, tubular structure that emerges from the basal mycelium.
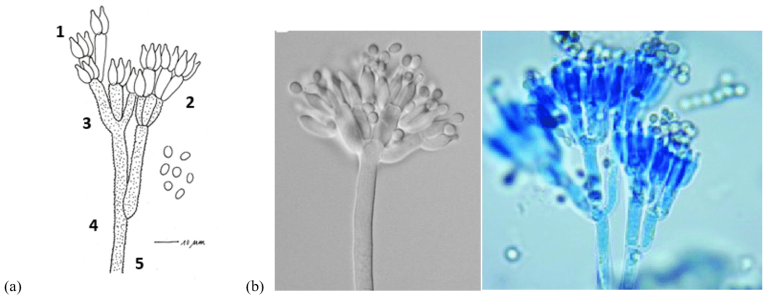
Image. 2 (a) Representation of the structure of Pensilium verrucosum: 1) Conidia, 2) Phialide, 3) Metulae, 4) Stalk, 5) Stipe (b) Images microscope Pensilium verrucosum
Ochratoxin production by Penicillium
Ochratoxin A, first isolated in South Africa in 1965 (van der Merwe et al., 1965), is mainly produced by the fungi Aspergillus and Penicillium. Within this latter specie, Penicillium verrucosum and Penicillium nordicum are the main producing species of Penicillium. These species are common in temperate and cold climates, developing mainly in cereals stored in conditions of inadequate humidity and temperature.
Certain abiotic factors favour their optimal levels in the production of mycotoxins:
- Water activity (aw): Levels between 0.80-0.85 are optimal for OTA production.
- Temperature: The production occurs between 4 °C and 31 °C, with an optimal range between 20-25 °C.
- pH and substrate: Cereals with a neutral pH and high carbohydrate content promote the production of mycotoxins.
Impact on feed and health
OTA is of particular concern in grains intended for human and animal consumption. Due to its thermal stability, it persists during processing, contaminating derivatives such as flour or animal feed. Chronic exposure to OTA has been associated in animals with toxic effects including weight loss, decreased feed efficiency and cumulative kidney damage.
Metabolism and mechanism of action
The greatest absorption of OTA takes place in the stomach and proximal jejunum. This absorption is high (40 to 60%) in pork and chicken. Ruminants absorb very little OTA, as the rumen flora rapidly transforms it to ochratoxin α (OTα) (Kuiper-Goodman et al., 1989, Petzinger et al., 2002). OTA has an efficient enterohepatic circulation (Kuiper-Goodman et al., 1989, Petzinger E, et al., 2002). In the bloodstream it is bound to albumin and other blood proteins (Galtier et al., 1998, Sreemannarayana et al., 1988).
Ochratoxicosis in animals
Porcine nephropathy is a non-infectious disease first described in Denmark in 1928 (Krogh P et al., 1991). At the beginning was associated with the consumption cereals with mold. Clinical signs in porcine nephropathy include polydipsia, polyuria, decreased production, depression, apathy and occasionally death (Cook WO et al., 1991).
OTA-associated avian nephropathy was first described in 1975 in Denmark. The birds’ kidneys were pale and larger than normal. Interstitial fibrosis and degeneration of the proximal and distal tubules of the nephrons were observed in these kidneys.
Metabolic pathways and OTA synthesis
Currently, more than 30 species of the genera Aspergillus and Penicillium are known producers of OTA (Ferrara et al., 2020; Perrone & Gallo, 2017; Wang et al., 2016). About 20 different analogues of OTs have been identified, however, ochratoxin A (OTA) is the most toxic derivative. This group was found to contain five highly conserved genes, encoding a PKS (otaA), an NRPS (otaB), a cytochrome P450 monooxygenase (otaC), a halogenase (otaD), and a basic leucine transcription factor (bZIP) (otaR1) In addition, genes encoding a flavin-adenine dinucleotide-dependent oxidoreductase (otaE) and a GAL4-like Zn2Cys6 binuclear DNA-binding protein (otaR2) have been described (Gallo et al., 2017; Wang et al., 2018b) (Fig. 1a).
The ochratoxin A (OTA) biosynthetic pathway begins with the PKS gene, otaA, which uses acetyl-CoA and malonyl-CoA to synthesize 7-methylmellein, which is then oxidized to beta-ochratoxin by otaC. The gene encoding NRPS, otaB, catalyzes the formation of the amide bond between beta-ochratoxin and beta-phenylalanine, resulting in the biosynthesis of ochratoxin B (OTB). Finally, OTB is chlorinated by otaD to produce OTA.
Regarding regulation: otaR1 acts as the specific regulator of the pathway, controlling the expression of the four biosynthetic genes (otaA, B, C and D). And otaR2 modulates only the expression of otaA, B and D (Ferrara et al., 2016; Gallo et al., 2012, 2014; Wang et al., 2018b) (Fig. 1b).
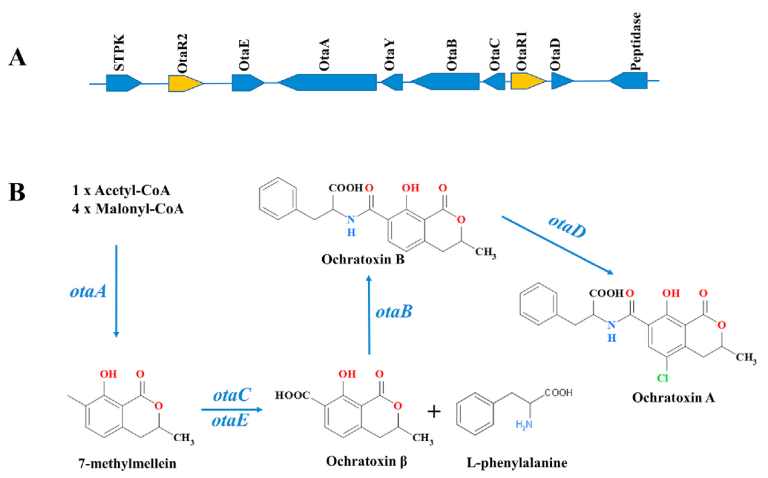
Figure 1. (A) Ochratoxins biosynthetic gene cluster in A. ochraceus. Arrowheads indicate the direction of gene transcription and the genes that regulate the cluster is indicated by the gold arrow. (B) Proposed biosynthetic pathway of ochratoxins (Ferrara et al., 2020; Wang et al., 2018b)
Prevention and Legislation
To minimize OTA contamination, it is recommended:
- Control of storage conditions: Keep the water activity low and a suitable temperature.
- Use of fungicides or biocontrol methods: Such as antagonistic bacteria or modified atmospheres.
- Legislation: European legislation establishes OTA recommendation values (EU 2016/1319) for products intended for animal feed.
| Raw material/feed | Recommended value in ppm with 12% humidity |
|---|---|
| Cereals and cereal-based products | 0.25 |
| Compound feed for pigs | 0.05 |
| Compound feed for poultry | 0.1 |
| Compound feed for cats and dogs | 0.01 |
Conclusion
The control of Penicillium in the feed chain is crucial to ensure food safety. A better understanding of the factors influencing OTA production allows for the development of more effective mitigation strategies and the protection of animal health.



