Mycotoxins are natural compounds produced as secondary metabolites by filamentous fungi, primarily by species like Aspergillus, Penicillium, and Fusarium, in a strain-specific manner (Egbuta et al ., 2017). These toxins contaminate around 25% of global food crops, although the actual contamination rate can range from 60.0% to 80.0% for certain mycotoxins, depending on various factors like the type of crop and specific mycotoxin (Eskola et al ., 2019).
Toxin-producing fungi can be divided into two categories: field fungi (e.g., Fusarium spp.), which infect crops during plant growth, and storage fungi (e.g., Aspergillus spp., Penicillium spp.), which predominantly contaminate crops after harvesting. Detecting these fungi in feed or raw materials doesn’t necessarily guarantee mycotoxin contamination. Multiple factors such as the fungal strain, substrate composition, moisture levels, aeration, and storage conditions influence the production of these toxic metabolites. Typically, hot and humid conditions are the primary factors promoting fungal growth and mycotoxin production (Saad et al ., 2016).
Mycotoxins in aqua feed
Mycotoxin contamination in fish feed can occur before or after harvest, especially in crops that are high in bran or fiber and have high moisture content. Post-harvest storage in unsuitable conditions, with increased temperature and water activity, can also promote mycotoxin production (Saad et al ., 2016). Currently, there are no methods to completely eliminate mycotoxins once they contaminate feed, but some processing techniques that use higher temperatures can help reduce mycotoxin levels (Bullerman et al ., 2016).
The use of plant-based materials in aquafeeds is on the rise. However, these ingredients are frequently contaminated with mycotoxin-producing fungi, notably Aspergillus flavus and Aspergillus parasiticus, which are widespread (Marijani et al ., 2019). Examples of mycotoxin contamination have been found in various countries, including Serbia, Brazil, Malaysia, and Portugal, in ingredients like corn, wheat, barley, soybean bran, and more (Gonçalves-Nunes et al ., 2016).
These mycotoxins found in feed ingredients have also been detected in finished aquafeed. For instance, in Brazil, aflatoxin B1 and fumonisins were detected in commercial feeds (Hashimoto et al ., 2016). In Nigeria, aflatoxin B1 was found in locally formulated fish feed, while Egypt saw aflatoxin B1 and ochratoxin A in their feed samples (Kholife et al ., 2016). Mycotoxins have been found in fish feed samples from various regions, with farm-made feed more commonly affected in developing countries (Mwihia et al ., 2018).
Mycotoxin contamination in fish feed is a widespread issue that varies by region. Aflatoxins are common in Southern Europe, Africa, South Asia, and Southeast Asia, while deoxynivalenol is more prevalent in North America, Northern and Central Europe, Africa, and North Asia. Zearalenone contamination is higher in North and South America, Central Europe, Africa, and North and Southeast Asia, and fumonisin is seen more in South America, Southern Europe, Africa, and various parts of Asia. Ochratoxin A is most prevalent in South Asia and Africa. Multi-toxin contamination is more common in Asia than in Europe or America, likely influenced by climate, sample types, and testing methods (Pinotti et al ., 2016).
Aflatoxins
Aflatoxins, the first mycotoxins discovered, gained attention after a severe outbreak of aflatoxicosis called “Turkey X disease” in the 1960s, which led to the death of about 100,000 turkeys. Among mycotoxins, aflatoxins are the most extensively studied and understood. The primary aflatoxins in crops are B1, B2, G1, and G2, mainly produced by A. flavus, though A. parasiticus and occasionally A. nomius can also synthesize them. Other filamentous fungi like Penicillium, Rhizopus, Mucor, and Streptomyces can produce aflatoxins as well. Aflatoxin B1’s precursor is sterigmatocystin, primarily produced by Aspergillus versicolor and Aspergillus nidulans, but other Aspergillus species and filamentous fungi can also create it.
A wide range of feed ingredients, including maize, corn, wheat, cottonseed, and nuts, can become contaminated with aflatoxins. However, the main sources of these toxins in animal feeds are groundnut meal, maize, and cottonseed meal. Sterigmatocystin, a precursor to aflatoxin, has been found to contaminate feed ingredients such as wheat, corn, maize, barley, and soybean. Contamination can occur both before and after harvest, with increased risk during plant stress conditions like high heat, drought, or insect infestation. Improper storage after fungal contamination can also lead to aflatoxin production. Elevated temperatures (above 27.0°C), humidity levels (above 62.0%), and feed moisture content (above 14.0%) contribute to increased synthesis of these fungal metabolites (Russo et al., 2013).
Various countries have established regulations for the maximum allowable levels of mycotoxins in food and feed, with differences arising from diverse dietary habits and crop consumption. In terms of feed materials, the maximum allowable level for aflatoxin B1 is 0.02 mg/kg, while for complete feed (except for cattle, sheep, goats, dairy animals, calves, lambs, pigs, and poultry, i.e., fish feed), it is 0.01 mg/kg. No specific regulations have been established for sterigmatocystin.
Aflatoxin B1 is a highly carcinogenic substance, known for its harmful effects on the liver and other organs. Besides being a carcinogen, it exhibits genotoxic and immunomodulatory effects on fish. Exposure to aflatoxin B1 results in stunted growth, anemia, blood clotting issues, liver damage, reduced immune function leading to susceptibility to infections, and increased mortality rates (Marijani et al., 2019).
Effects of aflatoxins on fish
Aflatoxicosis in fish leads to clinical signs such as pale gills, impaired blood clotting, slow growth, and inadequate weight gain. Severe infections may result in reduced survival rates, body discoloration (darkening/yellowing), and abnormal behavior, especially in species like the Nile tilapia and juvenile sturgeon (Marijani et al., 2019). The susceptibility of fish to aflatoxins varies with age and species, with fry being more vulnerable than older fish. Rainbow trout is highly sensitive to aflatoxins, with chronic exposure to low levels increasing the risk of cancer development. Even at a very low level of 0.0004 mg/kg, rainbow trout showed an elevated likelihood of developing liver tumors after 8 to 12 months of exposure. Aflatoxin B1 exposure also suppresses the immune system in trout. The LD50 (lethal dose for 50% of the population) for rainbow trout with aflatoxin B1 was determined to be 0.81 mg/kg (Bauer et al., 1969). Other sensitive fish species include sea bass and red drum .
Warmwater fish generally exhibit lower sensitivity to aflatoxins than freshwater fish. Channel catfish, for example, has an LD50 around ten times higher than that of rainbow trout. Nile tilapia is also less sensitive to aflatoxin B1 compared to rainbow trout, with an LD50 of 1.0 to 1.3 mg/kg, and their survival rate is not significantly affected by intake levels up to 3.0 mg/kg. However, the effects of aflatoxin B1 are dose- and duration-dependent (Deng et al., 2010).
Sterigmatocystin, a compound related to aflatoxins, causes similar health effects, primarily affecting the liver and kidneys. It is less toxic than aflatoxin but still leads to issues such as hepatocellular carcinoma in rainbow trout and DNA damage, chromosomal aberrations, increased micronucleated red blood cells, and histopathological lesions in the Nile tilapia. Sterigmatocystin exposure may also result in relatively high mortality rates. Both aflatoxins and sterigmatocystin can bioaccumulate in various fish species, with residues found in tissues like hepatopancreas, ovaries, muscles, liver, and more, although often in low concentrations. For example, sea bass was found to have approximately 0.005 mg/kg of aflatoxin B1 in its muscle tissue (El-Sayed et al., 2009). Sterigmatocystin residues were detected in the Nile tilapia’s edible tissues at around 8 µg/kg, but sterigmatocystin is considered to pose a lower health risk compared to aflatoxins.
Fumonisins
Fumonisin B1 is the most toxic type of fumonisin, primarily produced by several Fusarium species, with Fusarium verticillioides being a frequent producer. Alternaria alternata can also produce fumonisin B1. This mycotoxin is mainly found in maize and its by-products, with high contamination rates reported in countries like Mozambique, Burkina Faso, China, and Malaysia, where it has been detected in 80% to 100% of corn samples (Warth et al., 2012).
Regulations for maximum fumonisin levels in cereals mainly address co-contamination with fumonisin B1 and B2. The maximum allowed level for unprocessed maize (except maize intended for wet milling) is 4000.0 µg/kg. In feedstuffs, fumonisins should not exceed 60.0 mg/kg in maize and maize products, while complete fish feed should contain no more than 10.0 mg/kg.
Effects of fumonisins on fish
The effects of fumonisins on fish vary depending on the species. Channel catfish, for instance, exhibit moderate susceptibility. High doses of fumonisin B1 (>320 mg/kg) led to liver histological changes, growth suppression, and reduced survival, potentially due to impaired immune function. Concentrations of 20.0 mg/kg or more of fumonisin were associated with reduced growth rates and alterations in sphingolipid metabolism, possibly affecting cell health. Nile tilapia also displayed reduced growth rates and changes in sphingolipid metabolism when exposed to fumonisins (Tuan et al., 2003).
Common carp, on the other hand, showed high susceptibility to fumonisin-induced damage. Exposure to fumonisin B1 at levels of 10.0 mg/kg and 100.0 mg/kg for 42 days resulted in organ damage, affecting the liver, pancreas, kidney, heart, and brain, and causing blood vessel damage. In particular, the brain exhibited histopathological changes and vascular issues in young carp (Kovacic et al., 2009).
In general, fumonisins appear to induce organ damage, immune system impairment, reduced weight gain, metabolic changes that can lead to cancer, and increased mortality in fish. There are no reports of fumonisin accumulation in fish muscles, suggesting that fish consumption does not pose a significant food safety risk concerning this toxin.
Ochratoxin
Ochratoxin A, the most toxic among ochratoxins, is produced primarily by Penicillium spp. (Penicillium verrucosum) and Aspergillus spp., particularly Aspergillus ochraceous and Aspergillus carbonarius. Contamination by ochratoxin A typically occurs post-harvest in cereal grains like wheat, barley, oats, and corn, although it can affect other commodities as well. Ochratoxin A is known for its stability and resistance to elimination, making it difficult to remove from food products and allowing it to persist along the food chain.
European Commission regulations have established a maximum level of 5.0 µg/kg for ochratoxin A in unprocessed cereals and 3.0 µg/kg for all products derived from unprocessed cereals, including processed cereal products. In feedstuffs, cereals, and cereal products, the limit for ochratoxin A is set at 0.25 mg/kg. No specific limits have been set for complete animal feed other than for pigs and poultry, possibly due to limited knowledge about the effects of ochratoxins on other animals.
Effects of ochratoxin A on fish
Regarding the effects of ochratoxin A on fish, there is limited research, but fish species appear to be highly sensitive to this mycotoxin. For instance, adult sea bass exhibited high sensitivity, with an acute oral LC50 (96 h) of 277.0 µg/kg, leading to nervous and respiratory manifestations (El-Sayed et al., 2009). Juvenile catfish exposed to ochratoxin A (2.0 mg/kg and above) experienced reduced body weight gain, feed conversion, hematocrit levels, increased mortality, and kidney and liver lesions (Manning et al., 2003). Additionally, juvenile channel catfish exposed to ochratoxin A (4 mg/kg) showed increased mortality when infected with Edwardsiella ictalurid bacteria, likely due to a weakened immune response caused by ochratoxin A. In Nile tilapia, ochratoxin intoxication resulted in various effects, including sluggish swimming, reduced survivability, decreased growth, kidney and liver lesions, and decreased total protein levels (Diab et al., 2018).
The accumulation of ochratoxin in fish muscle does not appear to be significant in rainbow trout and Nile tilapia, and only low levels (mean 0.12 µg/kg) of ochratoxin A were found in the muscle of European seabass and gilthead seabream. This suggests that fish may not contribute significantly to the presence of ochratoxin A in the food chain.
Trichothecenes
Trichothecenes are mycotoxins produced in crops such as corn, wheat, barley, and oats by various fungi genera, including Fusarium, Myrothecium, Phomopsis, Stachybotrys, Trichoderma, and Trichothecium. The two most significant trichothecenes found in crops are deoxynivalenol and T-2 toxin. While T-2 toxin had significant effects on the health of zebrafish embryos, it does not seem to pose a substantial threat to fish or human health. On the other hand, deoxynivalenol (also known as vomitoxin) is produced mainly by Fusarium spp., particularly Fusarium graminearum, and is more common in feed than in food in Europe.
In feed materials, the maximum level for deoxynivalenol in maize by-products is 12.0 mg/kg, while for other cereals and cereal products, it is 8.0 mg/kg. In complete and complementary feedstuffs (excluding certain animals), deoxynivalenol should not exceed 5.0 mg/kg.
Effects of deoxynivalenol on fish
The effects of deoxynivalenol on fish are still not fully understood. Rainbow trout appears to be the most sensitive fish species, exhibiting reduced feed intake, weight gain, and growth rate when exposed to deoxynivalenol doses up to 2.6 mg/kg. Interestingly, deoxynivalenol seems to have a protective effect against bacterial infections in some fish species, such as channel catfish. In juvenile Atlantic salmon, exposure to deoxynivalenol led to reduced feed intake, weight gain, feed efficiency, and alterations in plasma protein and lipid levels, potentially affecting liver function and intestinal integrity.
While deoxynivalenol has been detected in the muscle of carp and rainbow trout, it usually accumulates at low levels. The mycotoxin is generally rapidly metabolized and does not tend to accumulate significantly in the organs of fish.
Zearalenone
Zearalenone is primarily produced by Fusarium spp., including F. graminearum, F. culmorum, F. equiseti, and F. crookwellense. Contamination by these fungi typically occurs before harvest in crops like corn. Zearalenone is known for its strong estrogenic activity, acting as a mycoestrogen that affects the reproductive abilities of various animals.
In feeds, maize byproducts should not exceed 3.0 mg/kg of zearalenone, while for other cereals and cereal products, the limit is 2.0 mg/kg. However, there are no established limits for complete feeds for animals other than mammals.
Effects of zearalenone on fish
The effects of zearalenone on fish are not fully understood. Rainbow trout, for example, can accumulate relatively high amounts of zearalenone in their ovaries (up to 7.1 µg/kg), but the impact on reproduction remains largely unknown. Short-term exposure of zebrafish to zearalenone has been shown to reduce spawning frequency and relative fecundity (Schwartz et al., 2013). Additionally, zearalenone at a concentration of 2.0 mg/kg appears to increase the growth rate and feeding efficiency of rainbow trout, but it may also have immunomodulatory effects that could potentially affect fish health (Wozny et al., 2019).
While zearalenone accumulates in the ovaries of rainbow trout and trace amounts have been detected in the liver and intestines, it does not seem to accumulate significantly in the muscle tissue of fish. Therefore, fish consumption does not appear to be a significant source of zearalenone exposure.
Multiple Mycotoxins Contamination
Fish feed can be exposed to multiple mycotoxins due to contamination in the raw materials or from different fungi producing various mycotoxins simultaneously. This can happen with Fusarium species, known for producing several toxins in the crops they infect. For instance, maize can be contaminated with both zearalenone and fumonisins, or with fumonisin and trichothecenes. Co-contamination can also occur when different fungi produce distinct mycotoxins. An example is sorghum, which can become contaminated with both Fusarium and Aspergillus, leading to the presence of fumonisin B1 and aflatoxin B1 after prolonged storage.
The effects of consuming feed and food contaminated with multiple mycotoxins are not well understood, particularly in relation to fish. More research, both in vitro and in vivo, is needed to comprehensively grasp their biological impact. When different mycotoxins are ingested simultaneously, they may interact with each other, potentially resulting in synergistic and/or additive effects, especially if their mechanisms of action are similar. For example, nephrotoxic mycotoxins like ochratoxin A and citrinin can have synergistic effects when consumed together. Similarly, the consumption of various trichothecenes, known as immunosupressants, can lead to additive effects in different animals. Consequently, fish contaminated with multiple mycotoxins could pose a particularly high risk to consumption.
Table 1. shows different studies on the effects of mycotoxins on various aquatic species.
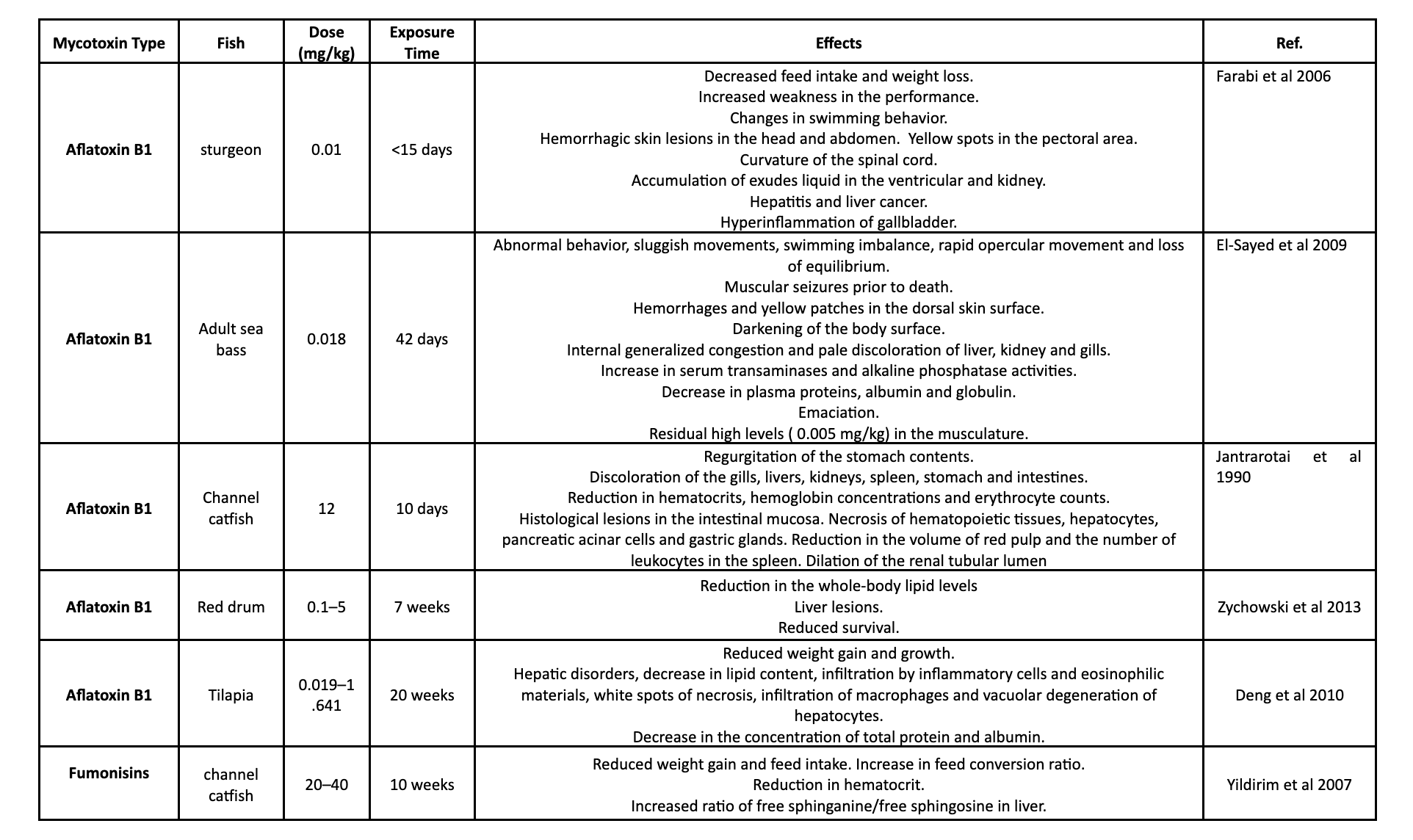
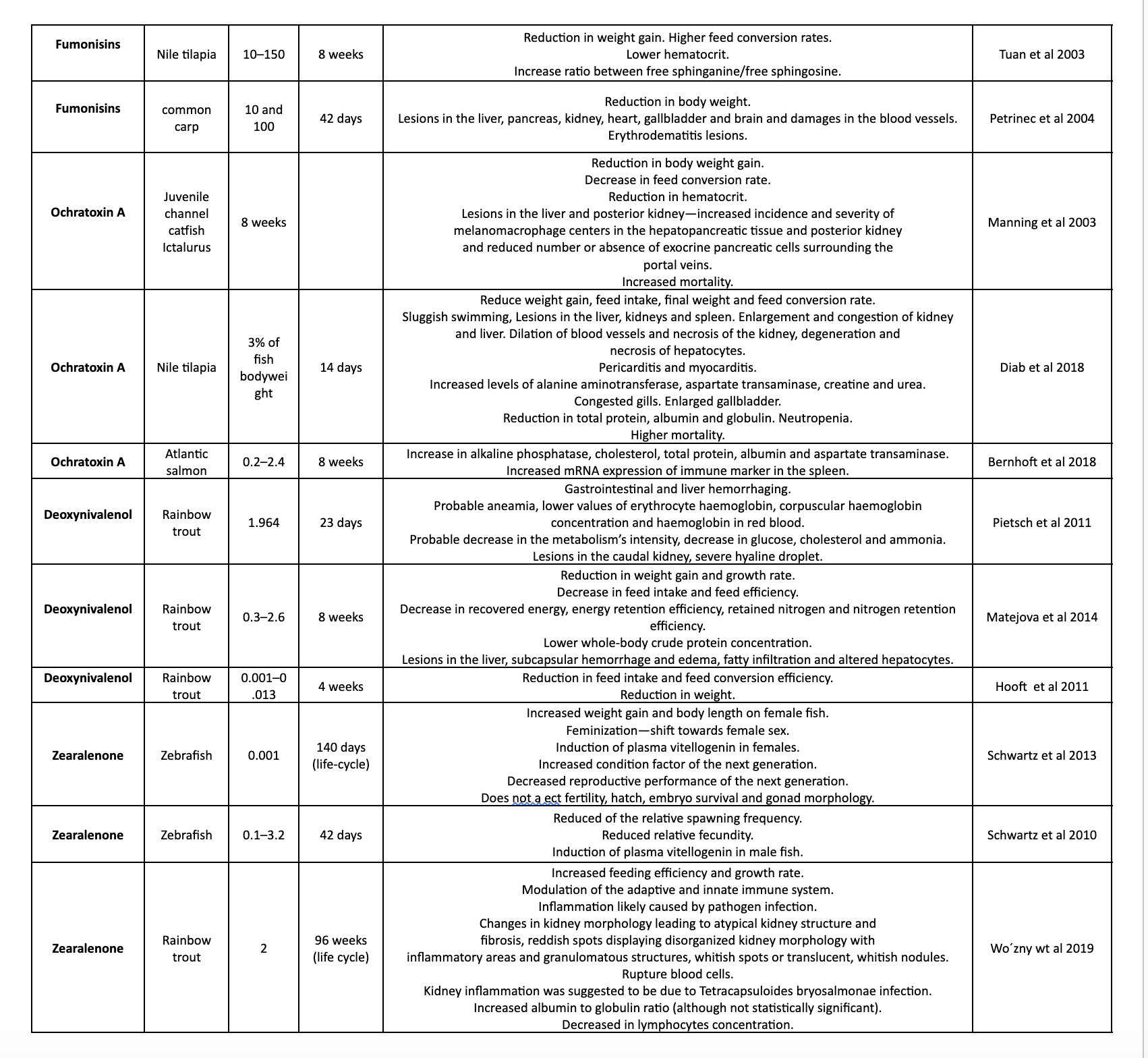
Conclusion
The use of plant-based ingredients in aquafeeds poses significant risks to aquaculture due to increased mycotoxin contamination. Fish consuming such feed suffer from higher disease rates, mortality, reproductive issues, and reduced weight gain, resulting in substantial economic losses. Even small mycotoxin traces in fish can endanger consumers’ health. This exacerbates existing mycotoxin exposure, especially in high-cereal consumption regions and developing countries, potentially leading to chronic health problems like cancer and weakened immune systems. Consequently, mycotoxins in aquafeeds have severe economic and public health consequences.
To combat this issue, proactive strategies for mycotoxin contamination control pre- and post-harvest are essential. Routine monitoring of raw ingredients and finished feed should become a standard practice to ensure the safety and sustainability of aquaculture’s growth in the future.



