Introduction
Mycotoxins are secondary metabolites produced by various fungi. Although they all fall into this category, their molecular structures exhibit significant differences that impact their chemical properties. Understanding these differences is crucial for addressing the different adsorption mechanisms.
One of the most relevant parameters historically used to evaluate the adsorption capacity of these substances is the chemical polarity, or simply polarity.
What is chemical polarity?
Chemical polarity is a property that describes the uneven distribution of electrical charges in a molecule. This characteristic is closely related to other physical and chemical properties, such as solubility, melting point, boiling point and intermolecular forces.
When a covalent bond is formed between atoms, the pair of electrons that forms the bond tends to move towards the atom that has the highest nuclear charge, that is, the one that has the most protons. This displacement generates an uneven distribution of the charge, forming an electric dipole. The greater the difference in electronegativity between the atoms involved in the bond, the more polar it will be. On the contrary, in bonds formed by equal atoms or with very small differences in electronegativity, the attraction of the electron pair is balanced, resulting from nonpolar molecules.
For example, water is a polar molecule. It is composed of oxygen and two hydrogens atoms, with different electronegativities (Image 01). The difference in electronegativity polarizes each H-O bond, shifting its electrons toward oxygen (illustrated by red arrows). These effects add up as vectors to make the overall molecule polar. The oxygen region indicated in red is the most electronegative part.
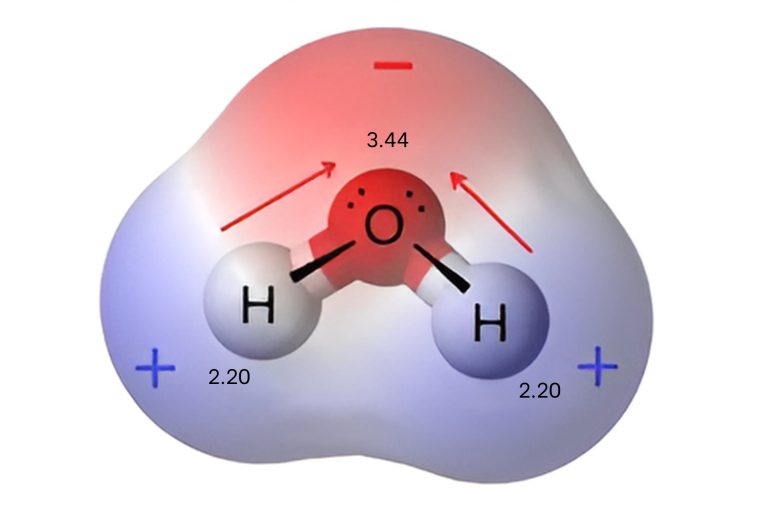
Image 1. Representation of electron density water molecule (H2O).
How to determine the polarity of a molecule?
Not all molecules containing polar bonds are entirely polarized. In fact, this depends on the number of polar bonds present and the spatial arrangement of the molecule. To determine whether a molecule is polar, the concept of electric dipole moment is used, which is defined as the vector magnitude that results from the product of the charge (q) times the distance (d) between the charges. The direction of this vector goes from negative to positive charge. The total polarity of the molecule is therefore the vector sum of the dipole moments of all the bonds present.
Molecular modeling enables the visualization of mycotoxin structures and the identification of areas with greater polarity. The following models represent the charge distribution of the main mycotoxins in order of polarity. The red zone indicates areas higher electronegativity, while the blue zone represents areas of higher electropositivity (see images below).
Aflatoxin B1
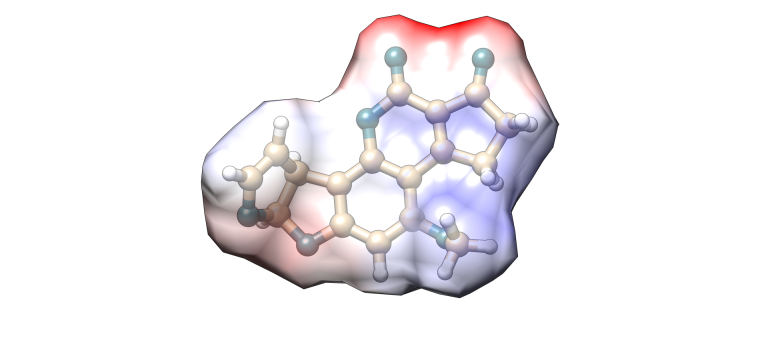
Image 2. Molecular model of Aflatoxin B1.
Fumonisin B1
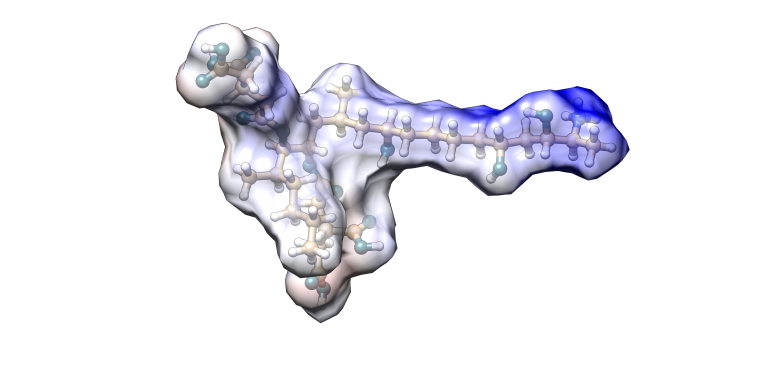
Image 3. Fumonisin B1 molecular model.
Ochratoxin A
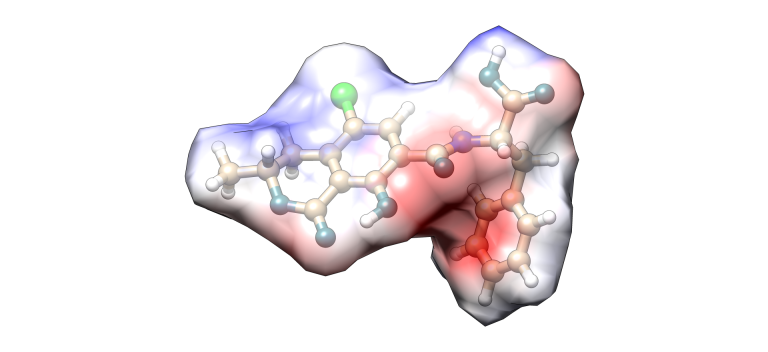
Image 4. Ochratoxin molecular model.
Trichothecenes
T-2 toxin
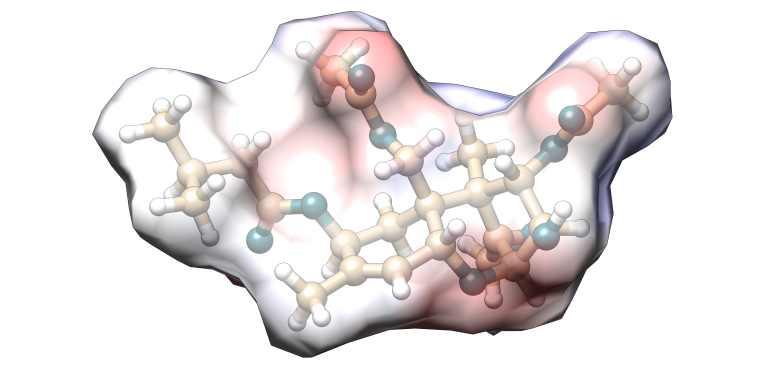
Image 5. Molecular model of T-2 toxin.
Deoxynivalenol (DON )
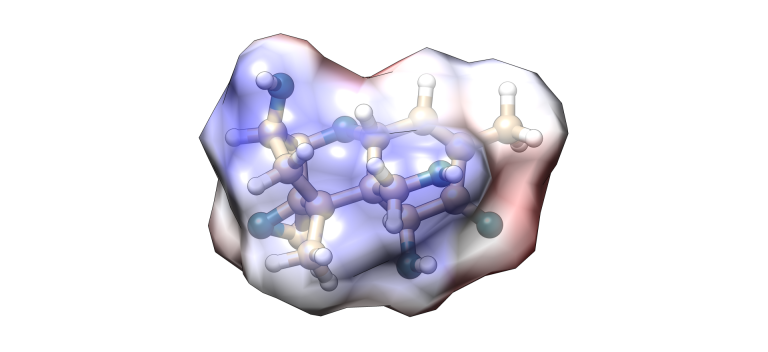
Image 6. DON molecular model.
Zearalenone
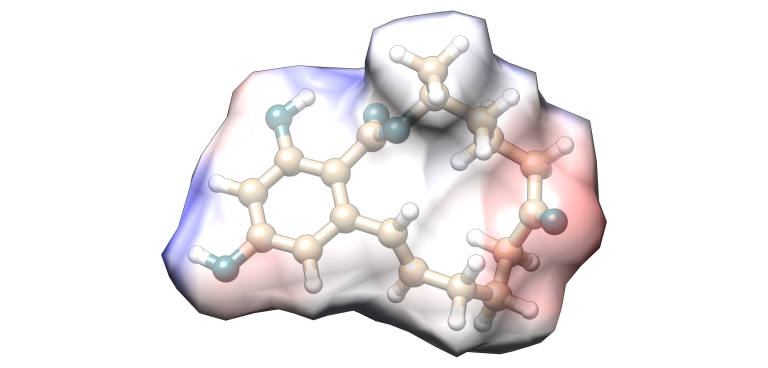
Image 7. Zearalenone molecular model.
Importance of polarity in mycotoxins
Polarity is a fundamental characteristic in the chemistry of mycotoxins, as it influences their behavior in various environments. This parameter not only helps to identify and classify different mycotoxins, but is also crucial to design effective strategies to mitigate their toxic effects. For example, polarity can determine the effectiveness of adsorbent products used to prevent the absorption of these substances into the body.
Although it is not possible to explain the effectiveness of adsorption only with the determination of polarity, it is very important to explain the behavior.
If we focus on the use of bentonites as aflatoxins adsorbents, these clays contain phyllosilicate minerals with a laminar structure that expands upon hydration, creating a characteristic interlayer space. This space is where aflatoxin can be introduced and retained, as electrostatic interactions enable the area with the highest charge (red color) to interact with the lamellae.
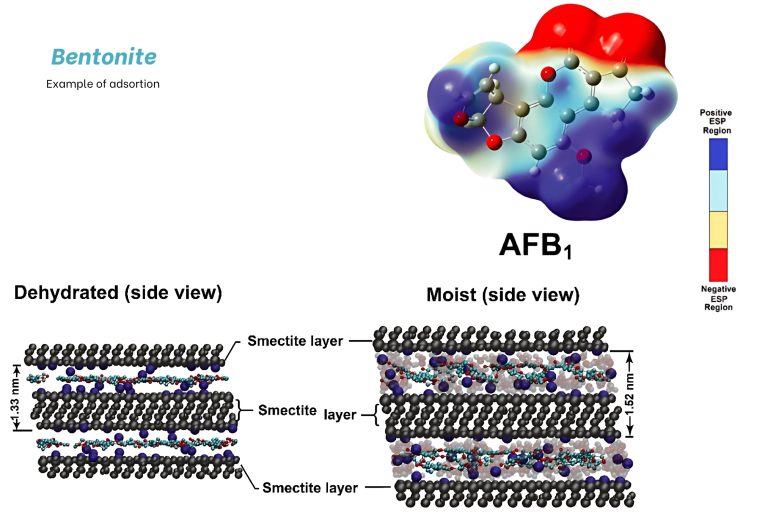
Image 8. Image represents the laminar structure of the phyllosilicate minerals in bentonites. Martínez et al. (2023).
Other factors that must be considered to understand the adsorption capacity of different materials for mycotoxins include the stereochemistry of mycotoxins. The shape and size of a mycotoxin can influence its ability to enter the interlayer spaces of bentonites, determining which type of bentonite is best suited for specific adsorption. To achieve optimal efficiency, it is essential to determine the ideal sheet separation that allows mycotoxins to be adsorbed effectively. If the separation is too small, mycotoxins will not enter; if it is too large, adsorption will be weaker, non-specific, and less effective. Another crucial factor is the contact capacity between mycotoxins and bentonites, which depends on optimizing the particle size of the bentonite to maximize adsorption. All these aspects are experimentally assessed through in vitro adsorption tests. Ensuring that the interlayer space is not excessively large is essential, as overly wide gaps lead to weaker or non-specific adsorption.
For example, the polar mycotoxin with the highest adsorption efficiency is aflatoxin. Its flat structure allows it to easily enter the interlayer spaces of bentonite minerals. Additionally, due to its electronegative zones, aflatoxin is a polar molecule that is effectively retained through electrostatic interactions.
These mycotoxin adsorption mechanisms, in addition to being carried out by bentonites, can also be performed by other phyllosilicate minerals, such as sepiolitic clays. In fact, one of the strategies often used is the combination of both types of clays, which can provide synergistic effects in the adsorption of different mycotoxins in a more efficient and specific manner. Furthermore, during formulation, other parameters are also considered to ensure proper storage and optimal product applicability, preventing the product from being too dusty or forming aggregates.
Conclusion
Understanding the chemical polarity of mycotoxins is crucial for comprehending the effects of adsorption. This knowledge enables the development of more effective strategies to mitigate the risks they pose, ensuring food safety and animal health. Research in this area not only provides essential insights into mycotoxins but also highlights the significant role of chemistry in understanding biological and environmental phenomena.



